|
|
V2
Bio-Consulting, Inc. is a specialized business providing cGMP and operational
expertise to the BioPharma Industry. Headquartered in the San
Francisco Bay Area, V2 Bio-Consulting seeks to address the special needs of
its clients from compliant strategic planning to tactical management. Our team of experienced Engineering, Project, Quality, Start-up, Commissioning
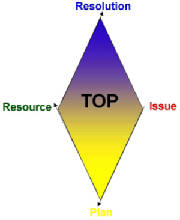
and Validation specialists provide "The Owner’s Perspective" (TOP)
to projects worldwide. V2 Bio-Consulting
extends your capabilities by partnering our specialists with your engineering and project teams to augment and optimize
your operational and/or project performance.
V2 Bio-Consulting's four-prong approach includes identifying the issues, devising plans to address the situation, and providing resources and the expertise to execute to resolution.
V2 Bio-Consulting, Inc. was founded in 2000. In response to the growing demand for services, the business expanded its staff and resources to offer a broader spectrum of BioPharma engineering and support capability. Our client base encompasses start-ups to established BioPharma leaders who value our expertise and results-oriented approach. Kurt Vorheis, principal and founder, has
over 25 years experience in the BioPharma Industry including VP of Manufacturing at Aviron and Sr. Director of Planning
and Director of Engineering at Chiron. Mr. Vorheis has extensive operational and project management experience moving
products and facilities from early clinical processes into large-scale commercial manufacturing. |
|||||||||||||